A Few Aldehydes
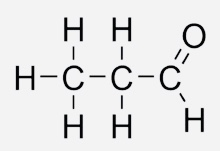
Acetaldehyde (also known as ethanal) is an organic compound which is widely occurs in nature and is also being produced on a large scale in industries. This chemical compound occurs naturally in bread, ripe fruit and coffee. Our liver also makes this compound while breaking down ethanol in our body.
CH3COH
Formaldehyde is a colourless gas with a very pungent and irritating smell. It is mostly sold in an aqueous form known as formalin, which is roughly 37% formaldehyde. It kills bacteria, even the healthy bacteria, and therefore it stops many of the important biological processes, causing tissue damage. It used in the manufacturing of Bakelite, which is a hard plastic having high electrical and chemical resistance.
HCOH
Benzaldehyde is an organic compound containing a benzene ring with a carbon in it attached to a formyl group.
A Few Ketones
Dimethyl ketone, also known as acetone is a colourless liquid which is used in nail polish removers, cellulose acetate, cellulose nitrate, acetylene and plastics.
CH3COCH3
Cyclohexanone contains six-carbon cyclic molecule with a ketone functional group. It is a colourless oil and an odor similar to that of acetone.
Article by Manth Kumar









0 Comments